Digital Pulseoximeter Including:
- Oxygen Saturation (SpO2)
- Pulse Rate (PR)
- Plethysmograph Waveform
- Perfusion Index (PI)
- Temperature High Accuracy and Precision of Parameters on Low Perfusion Condition
Setting for all Patient Type:
- Adult
- Pediatric
- Neonate
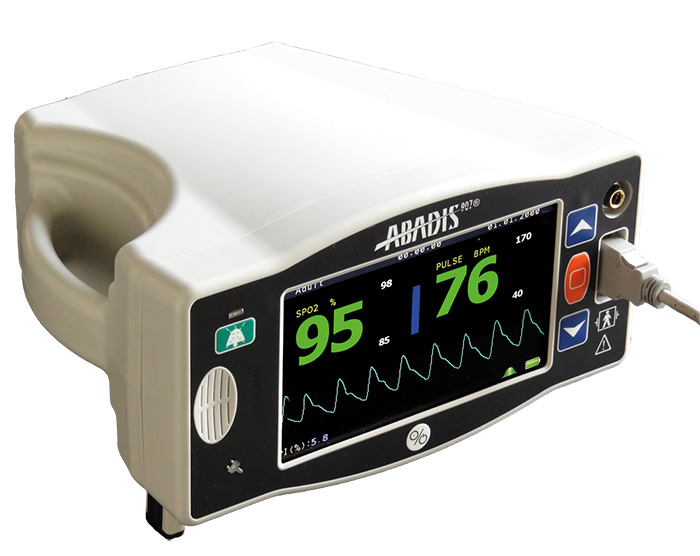
Technical Specification
- Internal Rechargeable Battery with 6 Hours Working Capacity
- ETHERNET Connector Complying HL7 Protocols
- USB Connector
- Arm Processor Design
- Unlimited Trend for Several Patients
- 7 Inch Full Color TFT LCD
- Auto-rotate Sensor for Detecting Horizontal or Vertical Positions
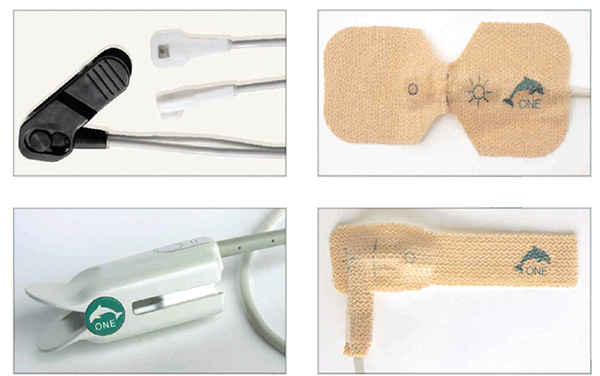
Oximetry performance is the most critical right when conditions are most difficult. That’s when you can count on
Dolphin Medical’s ONE™ (Oximetry Noise Elimination) technology.
Dolphin Medical’s ONE™ technology is the first and only system that uses a patented digital sensor – all others still use
an analog sensor.
Dolphin ONE™ sensors both amplify and digitize the signal in the sensor, resulting in a stronger and higher quality
signal. Dolphin’s OEM oximetry modules are designed to substantially improve performance and eliminate nuisance
alarms associated with motion and low perfusion. Dolphin’s OEM modules interface directly with the Dolphin ONE™
family of sensors and the host platform to-calculate the SpO2 and pulse rate.
| Electrical Characteristics | |
|---|---|
| Power Requirements (AC Line) | 100-240VAC; 50-60 Hz; 23-30VA |
| Battery | 7.6 VDC; Li-Ion, Rechargeable Maximum Protection Battery Operating Time: 4 hours Battery Charging Time: 4 hours |
| Fuse | 500 mA |
| Resolution | |
|---|---|
| SpO2 | 1% |
| Pulse Rate | 1 bpm |
| Temperature | 0.1°C |
| Sensor LEDs | |
|---|---|
| Nominal wavelenght and nominal power output values | (RED) 660 nm – 1.8 mw and (IRED) 905 nm – 2.0 mw |
| Battery | 7.6 VDC; Li-Ion, Rechargeable Maximum Protection Battery Operating Time: 4 hours Battery Charging Time: 4 hours |
| Alarm Limits | |
|---|---|
| Low SpO2 | 0% – 99% |
| High SpO2 | 1% – 100% |
| Low Pulse Rat | 30 bpm – 229 bpm |
| High Pulse Rate | 31 bpm – 230 bpm |
| Measurment Range | |
|---|---|
| SpO2 | 0% – 100% |
| Pulse Rate | 30 – 240 bpm (beat per minute) |
| Temperature | 0.0° – 60.0°C (32.0° – 138.9°F ) |
| Perfusion | 0.02% – 20% |
| Unit Dimensions & Weight | |
|---|---|
| Dimensions | 30 x 25 x 10 cm |
| Weight | 1.5 kg |
| Display | |
|---|---|
| Type | High Quality TFT Full Colors Liquid Crystal Display (LCD) with LED Backlight |
| Data Displayed | SpO2, Pulse Rate, Plethysmograph waveform, Temperature, Alarms and Status Message |
| Note: There is no display delay for the calculated value. | |
| Accuracy | ||
|---|---|---|
| SpO2 (Functional) | No Motion and Normal Perfusion | (70-100%) ±2% |
| Pulse Rate | No Motion and Normal Perfusion | (30-240 bpm) ±3 bpm |
| SpO2 (Functional) | Neonatal* No Motion and Normal Perfusion |
(70-100%) ±2% |
| SpO2 (Functional) | Motion, Low Perfusion <0.2% | (70-100%) ±3% |
| Pluse Rate | Motion, Low Perfusion <0.2% | (30-240 bpm) ±5 bpm |
| Temperature | Not Including Sensor Accuracy | (0-70°C) 1-2% |
| *Neonatal testing was completed on healthy adult subject and 1% was added to the % arms to account for fatal haemoglobin effects. | ||
| IEC (International Electrotechnical Commission) Classifications | |
|---|---|
| Type of Protection | CLASS II |
| Degree of Protection | Type BF |
| Mode of Protection | Continuous |
| Degree of Protection Against Ingress of Liquids | Ordinary (IPX2) |
| Recommended Methods of Sterilization or Disinfection | Using a soaked swab with alcohol for the external parts of system, SpO2 sensor and extension cable for appropriate cleaning instructions. |
| Degree of Safety of Application in the Presence of a Flammable Anesthetic | Not suitable for use in the presence of a flammable anesthetic mixture with air, oxygen or nitrous oxide. |
| Do not use functional tester to assess the accuracy of pulseoximeter probes and monitor. | |
| List of Relevant Standard | ||
|---|---|---|
| 1 | EN ISO 80601-2-61:2011 | Medical electrical equipment Part 2-61: Particular requirements for basic safety and essential performance of pulseoximeter equipment |
| 2 | EN 80601-1-8:2006 | Medical electrical equipment – Part 1-8: General requirements for safety – Collateral standard: 2. General requirements, tests and guidance for alarm systems in medical electrical equipment and medical |
| 3 | EN 60601-1:2006 | Medical electrical equipment – Part 1: General requirements for basic safety and essential performance |
| 4 | EN ISO 14971:2007 | Medical devices – Application of risk management to medical devices (ISO 14971:2007) |
| 5 | EN 60601-1-2:2001 | Medical electrical equipment – Part 1-2: General requirements for safety – Collateral standard: Electromagnetic compatibility – Requirements and tests |
| 6 | ASTM F2761-09 | Medical Devices and Medical Systems — Essential safety requirements for equipment comprising the patient-centric integrated clinical environment (ICE) — Part 1: General requirements and conceptual model |
Do You Have Any Question?
If You Have Any Question About ABADIS907 Please Ask Us.


